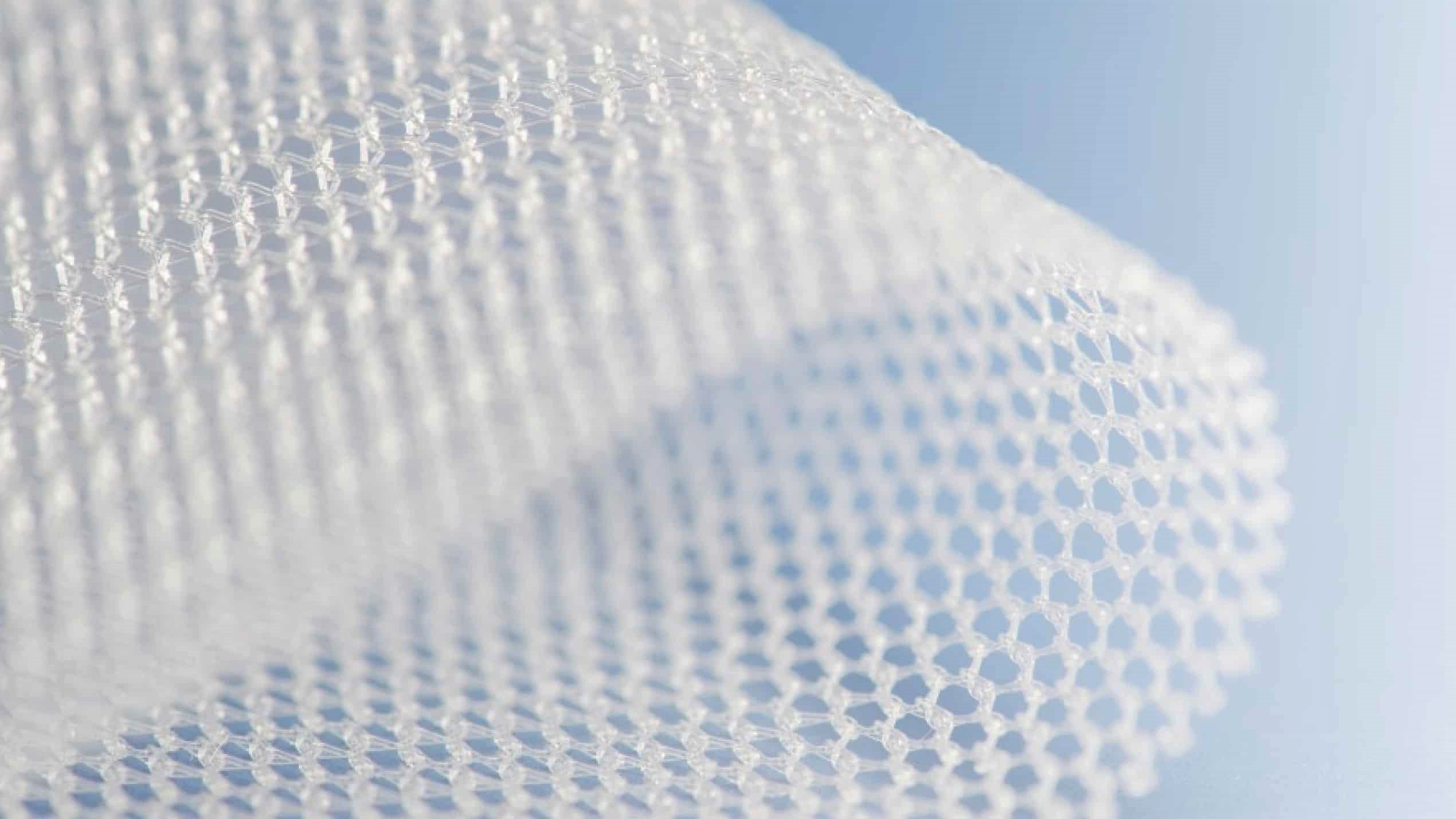
Hernia mesh is a surgical product used to stabilize abdominal tissues during a hernia repair surgery. It is used in about 90% of all hernia repairs which are performed about 800,000 times each year in the U.S. Unfortunately, thousands of these patients may experience severe side effects or complications as a result of the use of hernia mesh during their repair surgery.
Hernia mesh manufacturers including C.R. Bard, Atrium Medical and Ethicon, a division of Johnson & Johnson, may be facing more than 50,000 lawsuits for injuries caused by their devices.
Surgical mesh used for hernia repair surgery is a mesh-like device which may be constructed out of natural or synthetic materials like polypropylene, a type of plastic which may degrade after implantation. It is the same type of material used in other surgical repair products including many transvaginal mesh and bladder sling devices which have resulted in thousands of medical injury lawsuits.
In many cases, adverse events or complications have required additional surgeries to remove the defective device, repair the original hernia and repair or reconstruct damaged tissues. Additional surgeries place the patient at added risk and may require lengthy recovery periods.