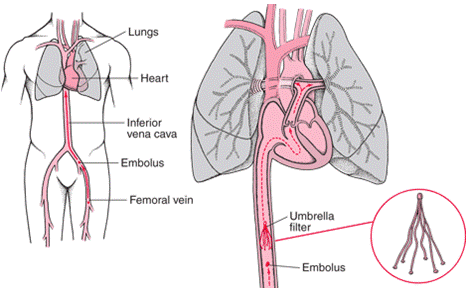
Inferior Vena Cava (IVC) filters are medical devices that are implanted in the Vena Cava and used to prevent blood clots from traveling into the lungs. IVC filters are typically used in patients with blood clotting disorders, like deep vein thrombosis (DVT), who are unable to take anticoagulant medication. The FDA has recommended that IVC filters be removed as soon as the blood clot passes, or the device may be more difficult or even impossible to retrieve – which can result in hemorrhaging, the development of more blood clots, severe pain and heart failure.
Several IVC filter manufacturers are facing lawsuits filed by patients who have experienced injury as a result of having the devices implanted. The lawsuits argue that companies marketed poorly designed devices and failed to properly warn of the potential risks involved with the filters.